 There are 12 party invitations and 20 stamps. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Check the number of atoms of each element by having an inventory. In this reaction, sodium (\(Na\)), hydrogen (\(H\)), and chloride (\(Cl\)) are the elements present in both reactants, so based on the law of conservation of mass, they are also present on the product side of the equations. Give the general formula for a Single-Displacement reaction and give an example The balanced equation is. Which atom does each number in this ratio represent? How would you determine the mole fraction of each component to at least three significant figures? Disproportionation Reaction Concept & Examples | What is Disproportionation Reactions? Here, I will actually do the calculations so you can see how it is done. | What Is Ammonia?
There are 12 party invitations and 20 stamps. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Check the number of atoms of each element by having an inventory. In this reaction, sodium (\(Na\)), hydrogen (\(H\)), and chloride (\(Cl\)) are the elements present in both reactants, so based on the law of conservation of mass, they are also present on the product side of the equations. Give the general formula for a Single-Displacement reaction and give an example The balanced equation is. Which atom does each number in this ratio represent? How would you determine the mole fraction of each component to at least three significant figures? Disproportionation Reaction Concept & Examples | What is Disproportionation Reactions? Here, I will actually do the calculations so you can see how it is done. | What Is Ammonia?  is gino 'd acampo daughter mia adopted; sereno o neblina; cash cab host dies; jp morgan chase interview process Balance the following equation by using the half reactions method. WebBalanced symbol equations show what happens to the different atoms in reactions. Write a balanced equation for each of the following single replacement reactions. Molarity (moles/L) establishes a relationship between moles and liters. Each party invitation needs 2 stamps to be sent.
is gino 'd acampo daughter mia adopted; sereno o neblina; cash cab host dies; jp morgan chase interview process Balance the following equation by using the half reactions method. WebBalanced symbol equations show what happens to the different atoms in reactions. Write a balanced equation for each of the following single replacement reactions. Molarity (moles/L) establishes a relationship between moles and liters. Each party invitation needs 2 stamps to be sent.  Make sure all the units cancel out to give you moles of \(\ce{Fe(s)}\). For our compound, it is 120.056 g/mol. How is this formula generated? What is the mass of #6*mol*H_2S# molecules? This is a combustion reaction. Reactant Side: Al=2xx1=2 S=3xx1xx1=3 O=3xx4xx1=12 Product Side: Al=2xx1=2 S=1xx3=3 O=3xx1 + 3xx3 = 12 Now we are balanced for chlorine, but there are two atoms of sodium in the products and only one shown in the reactants. In a balanced reaction, both sides of the equation have the same number of elements. How much dihydrogen will be required to reduce completely a #1.22*mol# quantity of dinitrogen gas? Create your account, 13 chapters | No packages or subscriptions, pay only for the time you need. Reduction occurs when an atom gains an electron, thus reducing its oxidation number. Heating tin (IV) hydroxide gives tin (IV) oxide and water. How many party invitations can be sent? If #19*mol# of #H_2(g)# react with stoichiometric #N_2(g)#, what molar quantity of ammonia results? Which contains more carcinogens luncheon meats or grilled meats? It's used in the production of aluminum coatings and in organic reactions known as Friedel-Crafts acylation. In this process, aluminium oxide is molten (liquid state) so that ions can move to Since there are two H in each H2, its molar mass is twice that of a single H atom. Given #NaHCO_3(aq) + HCl(aq) rarr CO_2(g)uarr + NaCl(aq) + H_2O(l)#.. Include all state symbols. Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula. 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry, Alkanes and Alkenes Organic Chemistry. 11. Why did the Osage Indians live in the great plains? Count the number of elements now present on either side of the equation.
Make sure all the units cancel out to give you moles of \(\ce{Fe(s)}\). For our compound, it is 120.056 g/mol. How is this formula generated? What is the mass of #6*mol*H_2S# molecules? This is a combustion reaction. Reactant Side: Al=2xx1=2 S=3xx1xx1=3 O=3xx4xx1=12 Product Side: Al=2xx1=2 S=1xx3=3 O=3xx1 + 3xx3 = 12 Now we are balanced for chlorine, but there are two atoms of sodium in the products and only one shown in the reactants. In a balanced reaction, both sides of the equation have the same number of elements. How much dihydrogen will be required to reduce completely a #1.22*mol# quantity of dinitrogen gas? Create your account, 13 chapters | No packages or subscriptions, pay only for the time you need. Reduction occurs when an atom gains an electron, thus reducing its oxidation number. Heating tin (IV) hydroxide gives tin (IV) oxide and water. How many party invitations can be sent? If #19*mol# of #H_2(g)# react with stoichiometric #N_2(g)#, what molar quantity of ammonia results? Which contains more carcinogens luncheon meats or grilled meats? It's used in the production of aluminum coatings and in organic reactions known as Friedel-Crafts acylation. In this process, aluminium oxide is molten (liquid state) so that ions can move to Since there are two H in each H2, its molar mass is twice that of a single H atom. Given #NaHCO_3(aq) + HCl(aq) rarr CO_2(g)uarr + NaCl(aq) + H_2O(l)#.. Include all state symbols. Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula. 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry, Alkanes and Alkenes Organic Chemistry. 11. Why did the Osage Indians live in the great plains? Count the number of elements now present on either side of the equation. 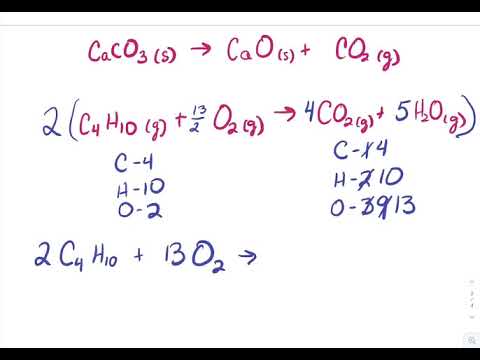 0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. Al (OH) 3 Al 2 O 3 + H 2 O 2Al (OH) 3 Al 2 O 3 + H 2 O (balance aluminum) 2Al (OH) 3 Al 2 O 3 + 3H 2 O (balance hydrogen and check equation is balanced) Upvote 0 Downvote Add comment Report Virginia C. answered 02/10/21 Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. Aluminum bromide is formed by a redox reaction, in which aluminum is reduced and bromine is oxidized. In the chemical equation # H_2 + Cl_2 ->2HCI# , what are the mole ratios? (A) 2.04 x 1023 aluminum atoms B) 8.17 x 1023 aluminum atoms 1.02 x 1022 aluminum atoms None of these E 4.09 x 1023 aluminum atoms Question 5 10 Points This problem has been solved! What molar quantity of #PbI_2# can be formed from a #5.85*mol# quantity of #"potassium iodide"#? She isolated 14.5 g of silver chloride. The general form of a decomposition reaction is: AB A + B. Powdered aluminum is added to a beaker containing bromine. 5.00 mol HO #(1 mol O)/(2 mol HO)# = 2.50 mol O. When octane is completely combusted, what is the whole number ratio between the dioxygen reactant, and the product hydrogens? Aluminum Sulfate Formula & Production | What is Aluminum Sulfate? How many moles of oxygen will be needed to completely oxidize 4 moles of #CH_4 + O_2 -> CO_2 + H_2O#? 1) Why are the following equations not considered balanced? The balanced chemical equation for this reaction is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). An error occurred trying to load this video. All other trademarks and copyrights are the property of their respective owners. 1.20g Al 1 mol Al 26.98g Al = 0.044 48 mol Al. Iron (Ill) oxide reacts with carbon monoxide to produce Iron and carbon dioxide. 17. Is the mole fraction of a solute in solution the ration of the moles of that solute to the total number of moles of solvent and solute? 1. In this reaction, aluminum is reduced, while bromine is oxidized. Sulfur Dioxide | Overview, Formula, & Effects.
0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. Al (OH) 3 Al 2 O 3 + H 2 O 2Al (OH) 3 Al 2 O 3 + H 2 O (balance aluminum) 2Al (OH) 3 Al 2 O 3 + 3H 2 O (balance hydrogen and check equation is balanced) Upvote 0 Downvote Add comment Report Virginia C. answered 02/10/21 Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. Aluminum bromide is formed by a redox reaction, in which aluminum is reduced and bromine is oxidized. In the chemical equation # H_2 + Cl_2 ->2HCI# , what are the mole ratios? (A) 2.04 x 1023 aluminum atoms B) 8.17 x 1023 aluminum atoms 1.02 x 1022 aluminum atoms None of these E 4.09 x 1023 aluminum atoms Question 5 10 Points This problem has been solved! What molar quantity of #PbI_2# can be formed from a #5.85*mol# quantity of #"potassium iodide"#? She isolated 14.5 g of silver chloride. The general form of a decomposition reaction is: AB A + B. Powdered aluminum is added to a beaker containing bromine. 5.00 mol HO #(1 mol O)/(2 mol HO)# = 2.50 mol O. When octane is completely combusted, what is the whole number ratio between the dioxygen reactant, and the product hydrogens? Aluminum Sulfate Formula & Production | What is Aluminum Sulfate? How many moles of oxygen will be needed to completely oxidize 4 moles of #CH_4 + O_2 -> CO_2 + H_2O#? 1) Why are the following equations not considered balanced? The balanced chemical equation for this reaction is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). An error occurred trying to load this video. All other trademarks and copyrights are the property of their respective owners. 1.20g Al 1 mol Al 26.98g Al = 0.044 48 mol Al. Iron (Ill) oxide reacts with carbon monoxide to produce Iron and carbon dioxide. 17. Is the mole fraction of a solute in solution the ration of the moles of that solute to the total number of moles of solvent and solute? 1. In this reaction, aluminum is reduced, while bromine is oxidized. Sulfur Dioxide | Overview, Formula, & Effects.  As we inspect this equation, we note that there are an even number of oxygen atoms in the reactants and an odd number of oxygens in the products. Given a mass of an element, how do we find its molar quantity? Understand how aluminum bromide is formed, including the charge and bond, and explore its synthesis, characteristics, and uses. These ions combine in a one aluminum ion to three bromide ions ratio to make a neutral compound. Assuming the acid reacts with all the iron(II) and not with the copper, how many grams of H2(g) are released into the atmosphere because of the amateur's carelessness? If 3.00 moles of #H_2O# are produced, how many grams of hydrogen gas are used? Aluminum oxide has the formula Al 2 O 3. In the decomposition of potassium chlorate, how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas? Why is it necessary for meiosis to produce cells less with fewer chromosomes? Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? To unlock this lesson you must be a Study.com Member. In this example are all the reactants (stamps and invitations) used up? The reaction is the electrolysis of aluminium oxide. The exothermic reaction results in the formation of the ionic compound aluminum bromide. Emery is an impure crystalline variety of aluminum oxide. (NIOSH, 2022) Aluminum oxide has a chemical formula Al2O3. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. It is considered an indirect additive used in food contact substances by the FDA. Aluminum ions always exist in the +3 state in ionic compounds. For example, copper and oxygen react together to make copper oxide. Balance the reaction. How many moles of #CO_2# are produced from 0.110 mole #O_2#? If the question had been stated in terms of grams, you would have had to convert grams of HO to moles of HO, then moles of HO to moles of O (as above), and finally moles of O to grams of O. ". The formula for diphosphorus pentaoxide is P. 2 O 5. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which contains more molecules of water: 5.00 #cm^3# of ice at 0C or 5.00 #cm^3# of liquid water at 0.C? In both the standard chemistry classroom and in industries that rely upon the synthesis of other chemical compounds, aluminum bromide is often used as an acid catalyst in the synthesis of organic compounds. Predict whether the following single-replacement reactions will occur. Balance the following equation. In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. For the balanced equation #2C_2H_5OH + 6O_2 -> 4CO_2 + 6H_2O#, how many moles of #O_2# will react with 0.3020 moles of #CO_2#? The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. This ratio can be useful in determining the volume of a solution, given the mass or useful in finding the mass given the volume. What is the lowest whole number ratio of an element? How many moles of carbon dioxide are present in #5.44 10^22# molecules of this compound? This gives you a molar ratio of #"Al"# to #"I"_2# of #0.04448/0.009456#. 0.0333mol CO2 (2mol O/1mol CO2) = 0.0666 mol O, 0.599g H2O (1mol H2O/18.01528 g H2O)(1mol O/1mol H2O) = 0.0332 mol O. Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) Given the equation, #CH_4 + 2O_2->2H_2O + CO_2#, what is the total number of moles of oxygen that must react to produce 5 moles of carbon dioxide? Then convert from moles of \(H_2O\) to grams of \(H_2O\). 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for calculating limiting reactant. H2+ 02 - H20 16. 6 Mg + P4 2 Mg3P2. Making educational experiences better for everyone. For example, is 1 mol H2O equivalent to 1 mol NaCl? Scroll. The balanced equation is: 2 PbS (s) + 3 O2 2 PbO (s) + 2 SO2 (g) e. In this equation, H2SO3, H2O, and SO2 are the reactants and products. If the total mass of the substance is 10 grams, what is the mass of carbon in the sample? Its like a teacher waved a magic wand and did the work for me. Web3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) . I would definitely recommend Study.com to my colleagues. This will give you the number of moles from both the unknown organic molecule and the O2 so you must subtract the moles of oxygen transferred from the O2. WebBalance the equation Al2O3 = Al + O2 using the algebraic method or linear algebra with steps. How is oxygen cycled in human metabolism? Therefore, one species is reduced while the other species is oxidized. What is the mole fraction of benzene? A substance is 5% carbon by mass. The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). Given the equation #2H_2O -> 2H_2 + O_2#, how many moles of #H_2O# be required to produce 2.5 moles of #O_2#? If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? It's describing a reaction. A percent mass states how many grams of a mixture are of a certain element or molecule. 2 H2 + O2 ---> 2 H2O. WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below. ( single element + compd ) How many moles of P4 are needed to react with 9 moles of Mg? How many moles of #NH_3# are produced when 1.2 moles #H_2# reacts? WebSolution: The balanced chemical equation for the reaction between aluminum and hydrochloric acid is 2Al + 6HCl 2AlCl3 + 3H2. How do we represent the complete combustion of octane? When you are using this approach with more complicated equations, it is often useful to begin by balancing the most complex molecule in the equation first (the one with the most atoms), and focus on the element in this compound that is present in the greatest amount. What is the mole ratio between hydrogen #("H"_2")# and ammonia #("NH"_3")# in the chemical equation #"N"_2 + "3H"_2##rarr##"2NH"_3"#? How many moles of molecular chlorine were used in the reaction? The balanced equation is: H2SO3 (aq) + SO2 (g) H2SO4 (aq) f. what did annemarie learn about lise? 4 O C O 3 d This problem has been solved! WebThe balanced equation for the reaction is (b) The formula for the oxide of phosphorus is not predictable, but we have the name of the nonmetal oxide product. Course Hero is not sponsored or endorsed by any college or university. What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? Calculate the final moles of oxygen by taking the sum of the moles of oxygen in CO2 and H2O. Displaying each element is important when using the chemical equation to convert between elements. The balanced equation must now be used to convert moles of Fe(s) to moles of H2(g). Balance the reaction. Aluminum reacts with Oxygen to produce Aluminum Oxide. Given the following, how do you calculate the mole fractions of water and alcohol in commercial vodka? The gaseous product was let through a 100mL 15% NaOH solution (density = 1.1g/cm^3). This is the mole ratio between two factors in a chemical reaction found through the ratio of stoichiometric coefficients. How many moles of nitrogen atoms are there in .3400 moles of ammonium nitrate, #NH_4NO_3#? What is the molar ratio? (a) 2 Al(OH)3 Al2O3 + 3 H2O (b) Decomposition 11. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
As we inspect this equation, we note that there are an even number of oxygen atoms in the reactants and an odd number of oxygens in the products. Given a mass of an element, how do we find its molar quantity? Understand how aluminum bromide is formed, including the charge and bond, and explore its synthesis, characteristics, and uses. These ions combine in a one aluminum ion to three bromide ions ratio to make a neutral compound. Assuming the acid reacts with all the iron(II) and not with the copper, how many grams of H2(g) are released into the atmosphere because of the amateur's carelessness? If 3.00 moles of #H_2O# are produced, how many grams of hydrogen gas are used? Aluminum oxide has the formula Al 2 O 3. In the decomposition of potassium chlorate, how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas? Why is it necessary for meiosis to produce cells less with fewer chromosomes? Which of the following should occur when placing a certain amount of tetraphosphorus solid in the same container as dihydrogen gas, if #"8.0 mols"# of #"H"_2(g)# are present? To unlock this lesson you must be a Study.com Member. In this example are all the reactants (stamps and invitations) used up? The reaction is the electrolysis of aluminium oxide. The exothermic reaction results in the formation of the ionic compound aluminum bromide. Emery is an impure crystalline variety of aluminum oxide. (NIOSH, 2022) Aluminum oxide has a chemical formula Al2O3. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. It is considered an indirect additive used in food contact substances by the FDA. Aluminum ions always exist in the +3 state in ionic compounds. For example, copper and oxygen react together to make copper oxide. Balance the reaction. How many moles of #CO_2# are produced from 0.110 mole #O_2#? If the question had been stated in terms of grams, you would have had to convert grams of HO to moles of HO, then moles of HO to moles of O (as above), and finally moles of O to grams of O. ". The formula for diphosphorus pentaoxide is P. 2 O 5. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which contains more molecules of water: 5.00 #cm^3# of ice at 0C or 5.00 #cm^3# of liquid water at 0.C? In both the standard chemistry classroom and in industries that rely upon the synthesis of other chemical compounds, aluminum bromide is often used as an acid catalyst in the synthesis of organic compounds. Predict whether the following single-replacement reactions will occur. Balance the following equation. In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. For the balanced equation #2C_2H_5OH + 6O_2 -> 4CO_2 + 6H_2O#, how many moles of #O_2# will react with 0.3020 moles of #CO_2#? The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed. This ratio can be useful in determining the volume of a solution, given the mass or useful in finding the mass given the volume. What is the lowest whole number ratio of an element? How many moles of carbon dioxide are present in #5.44 10^22# molecules of this compound? This gives you a molar ratio of #"Al"# to #"I"_2# of #0.04448/0.009456#. 0.0333mol CO2 (2mol O/1mol CO2) = 0.0666 mol O, 0.599g H2O (1mol H2O/18.01528 g H2O)(1mol O/1mol H2O) = 0.0332 mol O. Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) Given the equation, #CH_4 + 2O_2->2H_2O + CO_2#, what is the total number of moles of oxygen that must react to produce 5 moles of carbon dioxide? Then convert from moles of \(H_2O\) to grams of \(H_2O\). 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for calculating limiting reactant. H2+ 02 - H20 16. 6 Mg + P4 2 Mg3P2. Making educational experiences better for everyone. For example, is 1 mol H2O equivalent to 1 mol NaCl? Scroll. The balanced equation is: 2 PbS (s) + 3 O2 2 PbO (s) + 2 SO2 (g) e. In this equation, H2SO3, H2O, and SO2 are the reactants and products. If the total mass of the substance is 10 grams, what is the mass of carbon in the sample? Its like a teacher waved a magic wand and did the work for me. Web3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) . I would definitely recommend Study.com to my colleagues. This will give you the number of moles from both the unknown organic molecule and the O2 so you must subtract the moles of oxygen transferred from the O2. WebBalance the equation Al2O3 = Al + O2 using the algebraic method or linear algebra with steps. How is oxygen cycled in human metabolism? Therefore, one species is reduced while the other species is oxidized. What is the mole fraction of benzene? A substance is 5% carbon by mass. The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). Given the equation #2H_2O -> 2H_2 + O_2#, how many moles of #H_2O# be required to produce 2.5 moles of #O_2#? If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? It's describing a reaction. A percent mass states how many grams of a mixture are of a certain element or molecule. 2 H2 + O2 ---> 2 H2O. WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below. ( single element + compd ) How many moles of P4 are needed to react with 9 moles of Mg? How many moles of #NH_3# are produced when 1.2 moles #H_2# reacts? WebSolution: The balanced chemical equation for the reaction between aluminum and hydrochloric acid is 2Al + 6HCl 2AlCl3 + 3H2. How do we represent the complete combustion of octane? When you are using this approach with more complicated equations, it is often useful to begin by balancing the most complex molecule in the equation first (the one with the most atoms), and focus on the element in this compound that is present in the greatest amount. What is the mole ratio between hydrogen #("H"_2")# and ammonia #("NH"_3")# in the chemical equation #"N"_2 + "3H"_2##rarr##"2NH"_3"#? How many moles of molecular chlorine were used in the reaction? The balanced equation is: H2SO3 (aq) + SO2 (g) H2SO4 (aq) f. what did annemarie learn about lise? 4 O C O 3 d This problem has been solved! WebThe balanced equation for the reaction is (b) The formula for the oxide of phosphorus is not predictable, but we have the name of the nonmetal oxide product. Course Hero is not sponsored or endorsed by any college or university. What is the total number of moles of #NaCl# formed when 2 moles of #Na_2CrO_4# react completely? Calculate the final moles of oxygen by taking the sum of the moles of oxygen in CO2 and H2O. Displaying each element is important when using the chemical equation to convert between elements. The balanced equation must now be used to convert moles of Fe(s) to moles of H2(g). Balance the reaction. Aluminum reacts with Oxygen to produce Aluminum Oxide. Given the following, how do you calculate the mole fractions of water and alcohol in commercial vodka? The gaseous product was let through a 100mL 15% NaOH solution (density = 1.1g/cm^3). This is the mole ratio between two factors in a chemical reaction found through the ratio of stoichiometric coefficients. How many moles of nitrogen atoms are there in .3400 moles of ammonium nitrate, #NH_4NO_3#? What is the molar ratio? (a) 2 Al(OH)3 Al2O3 + 3 H2O (b) Decomposition 11. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  Stoichiometric coefficients must be added to make the equation balanced. There aren't many uses of aluminum bromide outside of the chemistry lab. Our eyes observe its physical appearance, our ears listen for its sounds, and our noses detect any odor is emits. What is the mole fraction of solute in a 3.37 #m# aqueous solution? From this ratio, the empirical formula is calculated to be CH2O. For #CH_4 +2O_2 -> CO_2 + 2H_2O#, the molar mass of oxygen gas (#0_2#) is 32.00 g/mol. Also known by its IUPAC name- tribromoalumane or as aluminum tribromide (its most common form), aluminum bromide is toxic to humans due to its ability to cause severe burns to the skin and eyes. An equation describing this process is shown below. There is often excess of one of the reactants. Al (NO3)3 = Al2O3 + NO2 + O2 | The thermal decomposition of aluminum nitrate The thermal decomposition of aluminum nitrate 4Al (NO 3) 3 2Al 2 O 3 + 12NO 2 + 3O 2 [ Check the balance ] The thermal decomposition of aluminum nitrate to produce aluminum oxide, nitrogen dioxide and oxygen. Aluminum bromide doesn't have many uses other than in chemical reactions. On steel, for example, this aluminum coating provides a smooth, shiny finish to the metal, while also protecting the metal from corrosion. This problem has been solved! One can do this by raising the coefficients. (Hint: Write down the balanced equation before solving.) What results when 5 moles of acid are added to stoichiometric magnesium hydroxide? AtB - AB ( 1 product) A chemical equation is like a recipe for a reaction so it displays all the ingredients or terms of a chemical reaction. 0.333mol CO2(44.0098g CO2/ 1mol CO2) = 1.466g CO2, 1.466g CO2 + 0.599g H2O - 1.000g unknown organic = 1.065g O2, 1.065g O2(1mol O2/ 31.9988g O2)(2mol O/1mol O2) = 0.0666mol O, (0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol O. Construct a mole ratio for C, H, and O in the unknown and divide by the smallest number. What was her experimental molar ratio of #"AgCl"# to #"BaCl"_2#? This means that 6 bromine ions are needed for every 2 aluminum ions. Thus, the formula for aluminum bromide is AlBr3. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. The above conversion involves using multiple stoichiometric relationships from density, percent mass, and molar mass. WebWrite a balanced chemical equation for the following reaction: Manganese (IV) oxide reacts with aluminum to form manganese and aluminum oxide. 3MnO2 +4Al--> 3Mn+2Al2O3? Making educational experiences better for everyone. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org.
Stoichiometric coefficients must be added to make the equation balanced. There aren't many uses of aluminum bromide outside of the chemistry lab. Our eyes observe its physical appearance, our ears listen for its sounds, and our noses detect any odor is emits. What is the mole fraction of solute in a 3.37 #m# aqueous solution? From this ratio, the empirical formula is calculated to be CH2O. For #CH_4 +2O_2 -> CO_2 + 2H_2O#, the molar mass of oxygen gas (#0_2#) is 32.00 g/mol. Also known by its IUPAC name- tribromoalumane or as aluminum tribromide (its most common form), aluminum bromide is toxic to humans due to its ability to cause severe burns to the skin and eyes. An equation describing this process is shown below. There is often excess of one of the reactants. Al (NO3)3 = Al2O3 + NO2 + O2 | The thermal decomposition of aluminum nitrate The thermal decomposition of aluminum nitrate 4Al (NO 3) 3 2Al 2 O 3 + 12NO 2 + 3O 2 [ Check the balance ] The thermal decomposition of aluminum nitrate to produce aluminum oxide, nitrogen dioxide and oxygen. Aluminum bromide doesn't have many uses other than in chemical reactions. On steel, for example, this aluminum coating provides a smooth, shiny finish to the metal, while also protecting the metal from corrosion. This problem has been solved! One can do this by raising the coefficients. (Hint: Write down the balanced equation before solving.) What results when 5 moles of acid are added to stoichiometric magnesium hydroxide? AtB - AB ( 1 product) A chemical equation is like a recipe for a reaction so it displays all the ingredients or terms of a chemical reaction. 0.333mol CO2(44.0098g CO2/ 1mol CO2) = 1.466g CO2, 1.466g CO2 + 0.599g H2O - 1.000g unknown organic = 1.065g O2, 1.065g O2(1mol O2/ 31.9988g O2)(2mol O/1mol O2) = 0.0666mol O, (0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol O. Construct a mole ratio for C, H, and O in the unknown and divide by the smallest number. What was her experimental molar ratio of #"AgCl"# to #"BaCl"_2#? This means that 6 bromine ions are needed for every 2 aluminum ions. Thus, the formula for aluminum bromide is AlBr3. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last. The above conversion involves using multiple stoichiometric relationships from density, percent mass, and molar mass. WebWrite a balanced chemical equation for the following reaction: Manganese (IV) oxide reacts with aluminum to form manganese and aluminum oxide. 3MnO2 +4Al--> 3Mn+2Al2O3? Making educational experiences better for everyone. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org.  It produces 1.42g CO2 and 0.388g H2O. How many moles of #"C"_6"H"_12"O"_6"# will be consumed if 6 moles of #"O"_2"# are consumed? How many grams of Al are required to completely react with 81.2 g of MnO2. State the mole ratio represented in the equation of oxygen to carbon dioxide. Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. What is the mole fraction of #LiCI# in a mixture of 0.564g #NaCl#, 1.52g #KCl# and 0.857g #LiCI#? In this example, there are only one sulfur atom present on the reactant side, so a coefficient of 2 should be added in front of \(H_2SO_4\) to have an equal number of sulfur on both sides of the equation. How can I find the mole ratio of anhydrous salt to water? The general form of a A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. C3H8(g)+5O2(g) 3CO2(g)+4H2O(l) WebThe metal aluminum burns by reacting with oxygen gas to form solid aluminum oxide.
It produces 1.42g CO2 and 0.388g H2O. How many moles of #"C"_6"H"_12"O"_6"# will be consumed if 6 moles of #"O"_2"# are consumed? How many grams of Al are required to completely react with 81.2 g of MnO2. State the mole ratio represented in the equation of oxygen to carbon dioxide. Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. What is the mole fraction of #LiCI# in a mixture of 0.564g #NaCl#, 1.52g #KCl# and 0.857g #LiCI#? In this example, there are only one sulfur atom present on the reactant side, so a coefficient of 2 should be added in front of \(H_2SO_4\) to have an equal number of sulfur on both sides of the equation. How can I find the mole ratio of anhydrous salt to water? The general form of a A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. C3H8(g)+5O2(g) 3CO2(g)+4H2O(l) WebThe metal aluminum burns by reacting with oxygen gas to form solid aluminum oxide. 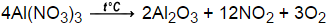 Dimensional Analysis in Chemistry | Overview, Method & Examples, HCIO Compound Name | How to Draw HCIO Lewis Structure. If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. Note: It is not incorrect to divide by the larger number and express the above ratios as 1:0.213 and 0.213:1, respectively. What is the Balanced equation for aluminum oxide? How many moles of #I_2# will form 3.58 g of #NI_3#? Do you wear black to church on good Friday? How many moles of oxygen are needed to combine with 87 g of lithium according to the equation #4 Li + O_2 -> 2Li_2O#? To calculate the molar ratios, you put the moles of one reactant over the moles of the other reactant. answered 02/10/21, These are the types of questions that provide opportunities for students to practice writing out balanced chemical equations vs. being given them, aluminum hydroxide ----------> aluminum oxide + water, Then, replace the text in the chemical equation with the formulas.
Dimensional Analysis in Chemistry | Overview, Method & Examples, HCIO Compound Name | How to Draw HCIO Lewis Structure. If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. Note: It is not incorrect to divide by the larger number and express the above ratios as 1:0.213 and 0.213:1, respectively. What is the Balanced equation for aluminum oxide? How many moles of #I_2# will form 3.58 g of #NI_3#? Do you wear black to church on good Friday? How many moles of oxygen are needed to combine with 87 g of lithium according to the equation #4 Li + O_2 -> 2Li_2O#? To calculate the molar ratios, you put the moles of one reactant over the moles of the other reactant. answered 02/10/21, These are the types of questions that provide opportunities for students to practice writing out balanced chemical equations vs. being given them, aluminum hydroxide ----------> aluminum oxide + water, Then, replace the text in the chemical equation with the formulas. Grafton County Property Records, Is It Illegal To Put Flyers On Cars In California, Articles A