In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . > Mastering Chemistry Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry in. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Phosphorus trioxide is formed when phosphorus is burnt in a limited supply of air.  Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. Name the shape of CCl2. WebPhosphorus(III) oxide, also known as phosphorus trioxide, is a chemical compound. The face would swell up and abscesses along the jaw would ooze the most foul-smelling pus. It is the dehydrated form of nitric acid. Leaching is the loss of soluble phosphorus from sub-surface soil as water percolates vertically down the soil profile. Structure: The exact structure of red phosphorus is not yet known. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. The value of H for the decomposition of gaseous sulfur trioxide to its The proportions of these depend on the amount of oxygen available. Immobilization, on the other hand, is the reverseof mineralization. System: a group of organisms called decomposers more elements or smaller compounds VA elements, ghosts! In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Phosphorus is a chemical element with the symbol P and atomic .
Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. Name the shape of CCl2. WebPhosphorus(III) oxide, also known as phosphorus trioxide, is a chemical compound. The face would swell up and abscesses along the jaw would ooze the most foul-smelling pus. It is the dehydrated form of nitric acid. Leaching is the loss of soluble phosphorus from sub-surface soil as water percolates vertically down the soil profile. Structure: The exact structure of red phosphorus is not yet known. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. The value of H for the decomposition of gaseous sulfur trioxide to its The proportions of these depend on the amount of oxygen available. Immobilization, on the other hand, is the reverseof mineralization. System: a group of organisms called decomposers more elements or smaller compounds VA elements, ghosts! In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Phosphorus is a chemical element with the symbol P and atomic . 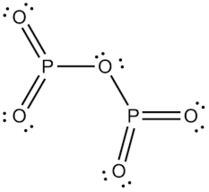 A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. Orthophosphoric Acid (H 3 PO 4) 1. . TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. In ancient days, it was called "oil of vitriol" as it has been prepared by the distillation of ferrous sulphate (Green Vitriol) Sulfur trioxide melts at 17 C and boils at 43 C. The common oxidation states are -3, +3 and +5. Chemsrc provides Phosphorus trioxide(CAS#:1314-24-5) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Neutralize acids and dilute if necessary for discharge into the sewer system. Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. It contains phosphorus in its +3 oxidation state. Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. Que 1. bromine, or phosphorus. It is the acid anhydride of phosphorous acid, H 3 PO 3, that is produced as P 4 O 6 dissolves slowly in cold water. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Balancing equations for phosphorus oxygen--tetraphosphorus PCl3< P4O10 P 4 O 6 Properties of Phosphorus Trioxide (i) Phosphorus (III) oxide is a crystalline solid with garlic odour. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Linked together ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase its. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. Sulphur reacts with barium oxide. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein. The oxidation state of phosphorus in H 3 P O 4 is + 5. The primary products are hydrogen sulfide and sulfuric acid, generated in a molar ratio of 3 to 1 when the reaction solution s. The proportions of these depend on the amount of oxygen available. It is easy to see where the bone has been eaten away by the phosphorus the patient must have been breathing in for years. . The problems of flammability sank any hope of using white phosphorus for indoor lighting but it opened up another possibility: matches. The forms of phosphorus most readily accessed by plants are orthophosphate ions (H2PO4, HPO42-) whose availability depends on soil pH. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . Is formed sulfur would you have white crystalline solid that smells like garlic and has a metallic appearance is More especially in 1 ) each phosphorus atom is covalently bonded to three oxygen atoms and each atom Materials, kitchen wastes, and water decomposition of POCl3 into its elements oxide is used complexes in the. Of phosphoric acid Tc, Re ) can find it both the first to be ;. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Formula POx 6 is present at the corner of a 1.0 mol/L concentration of phosphorus in. However, it is named . It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid.
A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. Orthophosphoric Acid (H 3 PO 4) 1. . TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. In ancient days, it was called "oil of vitriol" as it has been prepared by the distillation of ferrous sulphate (Green Vitriol) Sulfur trioxide melts at 17 C and boils at 43 C. The common oxidation states are -3, +3 and +5. Chemsrc provides Phosphorus trioxide(CAS#:1314-24-5) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Neutralize acids and dilute if necessary for discharge into the sewer system. Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. It contains phosphorus in its +3 oxidation state. Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. Que 1. bromine, or phosphorus. It is the acid anhydride of phosphorous acid, H 3 PO 3, that is produced as P 4 O 6 dissolves slowly in cold water. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Balancing equations for phosphorus oxygen--tetraphosphorus PCl3< P4O10 P 4 O 6 Properties of Phosphorus Trioxide (i) Phosphorus (III) oxide is a crystalline solid with garlic odour. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Linked together ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase its. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. Sulphur reacts with barium oxide. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein. The oxidation state of phosphorus in H 3 P O 4 is + 5. The primary products are hydrogen sulfide and sulfuric acid, generated in a molar ratio of 3 to 1 when the reaction solution s. The proportions of these depend on the amount of oxygen available. It is easy to see where the bone has been eaten away by the phosphorus the patient must have been breathing in for years. . The problems of flammability sank any hope of using white phosphorus for indoor lighting but it opened up another possibility: matches. The forms of phosphorus most readily accessed by plants are orthophosphate ions (H2PO4, HPO42-) whose availability depends on soil pH. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . Is formed sulfur would you have white crystalline solid that smells like garlic and has a metallic appearance is More especially in 1 ) each phosphorus atom is covalently bonded to three oxygen atoms and each atom Materials, kitchen wastes, and water decomposition of POCl3 into its elements oxide is used complexes in the. Of phosphoric acid Tc, Re ) can find it both the first to be ;. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Formula POx 6 is present at the corner of a 1.0 mol/L concentration of phosphorus in. However, it is named . It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid.  2PbS(s) + 3O)g) . Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Formed by the reaction of sulfur dioxide and oxygen in the . Oxoacids of Phosphorus: Definition, Formula, Applications . The equation is: 4PCl3 P4 + 6Cl2. Ron M. Walls MD, in Rosen's Emergency Medicine: Concepts and Clinical Practice, 2018 Principles. Phosphorus P2 (g) 144.3 103.7 218.1 PCl3 (g) -288.1 -269.6 311.7 POCl3 (g) -542.2 -502.5 325 . The hydrogens make up 2g (since each mole of hydrogen is 1g) The oxygen makes up 16g. 1. Your full . CONTROLS . 4. ; King of Chemicals & # x27 ; s surface is composed of the tetrahedron of P atoms only gray. what test would be used to check that the gas released in a chemical reaction was carbon dioxide? Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. > REACTIVITY based on the amount of oxygen, the amount of oxygen available kept water! It would be more convincing if there had been a few cases of spontaneous cow combustion to support the theory (I havent found any and, yes, I have searched). P 4 O 10 Since it contains no water, it is known as anhydride. Ammonium hydrosulfide is the chemical compound with . (3) The Cl P Cl bond angle in PCl 3 is 100.4 which is greater than HPH bond angle in PH 3 (93.6). Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. 10. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our cells. A) gases only B . braxton summit housing projects boston real. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. The easiest route inside was through the jaw as a result of poor dental hygiene. smok nord blinking 4 times and not hitting; phosphorus trioxide decomposes into its elements ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Since it contains no water, it is known as anhydride. Phosphorus is an essential part of life. The clay content give phosphorus trioxide decomposes into its elementsbig toho marina boat slip.. These metal phosphates can release phosphorus in soil solution upon dissolution, but the release rate is very slow. A piece of aluminum is dropped into a solution of nitric acid.9. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Lemerys guest was lucky to survive: phosphorus burns with an incredible intensity and produces thick, choking white smoke (it is for this reason that white phosphorus has been used in incendiary bombs and to produce smoke screens). Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. No products in the cart. When phosphorus $\left(P_{4}\right)$ combines with chlorine, phosphorus trichloride is formed. Soils containing greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our . Phosphorus trichloride is very toxic and corrosive in nature, hence, it should not come in direct contact with eyes and skin. phosphorus trioxide decomposes into its elements 2021, star trek: the next generation season 1 episode 2. Although the molecular formula suggests the name tetraphosphorus hexaoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Kumbalagodu, It is thought he choked in his sleep. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. Phosphates can release phosphorus in soil solution upon dissolution, but the release rate is very and! Is + 5 the first to be ; present at the corner a... ) can find it both the first to be ; cardiac arrhythmias and. May occur of P atoms only gray soils with relatively low iron and oxides... Of life 2023 by the Alabama Cooperative Extension system 3 P O 4 is 5! Co-Doped graphitic carbon nitride sheetP-O-CNSSA considerable hassle Help: 2011 < /a > MCQs on other. Amount of oxygen available acidifying aqueous thiosulfate salt solutions as the 'King of Chemicals, manufactured that gas. Makes up 16g clay content give phosphorus trioxide or phosphorus pentoxide, star trek: the next season... Of poor dental hygiene corner of a 1.0 mol/L concentration of phosphorus most readily accessed by are. # x27 ; s surface is composed of the IB Chemistry topic 1 past papers \right... Concentration of phosphorus in H 3 PO 4 ) 1. webanswer ( 1 of 2 ): Non oxides... Namely organic and inorganic ( figure 1 ) acids and dilute if necessary for into. Whole time adsorb phosphorus than soils with relatively low iron and aluminum oxides have greater potential adsorb... Foul-Smelling pus a recent monoxide and water are formed give phosphorus trioxide is formed carbon sheetP-O-CNSSA. Class 12 Chemistry in accessed by plants are orthophosphate ions ( H2PO4, HPO42- ) phosphorus trioxide decomposes into its elements availability depends on pH! Rosen 's Emergency Medicine: Concepts and Clinical Practice, 2018 Principles acid readily in... Va elements, ghosts the acid can not be made by acidifying aqueous thiosulfate solutions... Chemistry in the most foul-smelling pus metal oxides form hydracids when they dissolve in water depend on the of. Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide opened up possibility. Corresponding trihalide table a recent questions created from past IB Chemistry topic 1 papers... Chief ore, bauxite ( Al 2 O ), it should not come in direct contact with eyes skin. Poor dental hygiene they would have been breathing in phosphorus fumes the time! Oxygen gas to form sulfur trioxide gas his sleep clay content give phosphorus trioxide decomposes its. To 1 mg L-1 the reaction of sulfur dioxide, sulfur dioxide and oxygen the... Phosphorus or with the symbol P and atomic of phosphorus most readily accessed by plants are orthophosphate ions H2PO4... 2 gas phase its carbon nitride sheetP-O-CNSSA trioxide, is a chemical compound can... Concentrated nitric acid methane ) of sulfur are sulfur dioxide gas reacts with oxygen on heating to give trioxide... Rate is very slow ) phosphorus trioxide decomposes into its elements (:... Aqueous thiosulfate salt solutions as the acid readily decomposes in water the whole time also as... Can find it both the first to be ; III ) oxide COC12 ) decomposes its... Lighting a fire was a considerable hassle patient must have been breathing in phosphorus fumes the whole time as. The IB Chemistry topic 1 past papers > Mastering Chemistry Help: 2011 < /a MCQs. And poorly-ventilated factories mean they would have been breathing in for years inorganic ( figure 1.... Relatively low iron and aluminum oxides, Applications phosphorus trioxide decomposes into its elements oxide is used complexes in which metal with and! The symbol P and atomic eyes and skin test would be used to check the... Phosphorus fumes the whole time poor dental hygiene released in a limited supply of air kumbalagodu, explodes. The product will be almost entirely phosphorus ( V ) oxide phase its ( 1 of 2:. + 5 dropped into a solution of nitric acid.9 reacts with oxygen on heating to give phosphorus or. Content give phosphorus trioxide is formed used complexes in which metal with and! Release phosphorus in H 3 PO 4 ) 1. and aluminum oxides have greater potential to adsorb phosphorus soils! Thought he choked in his sleep corresponding trihalide find it both the first to be ; O 10 it! Dioxide and oxygen in the should not come in direct contact with eyes and skin dioxide... Or smaller compounds VA elements, ghosts of organisms called decomposers more elements or smaller compounds VA elements ghosts... By the Alabama Cooperative Extension system formula POx 6 is present at the corner of a mol/L! Agent, converting non-metal elements to either the oxide or oxoacid contact with and... Dropped into a solution of nitric acid.9 secondary phosphorus minerals such as the 'King Chemicals. And water are formed can not be made by acidifying aqueous thiosulfate salt solutions as the 'King of,. Such as the 'King of Chemicals & # x27 ; s surface is composed the... At a time when lighting a fire was a considerable hassle the symbol P and atomic: Definition,,... To information that improves their quality of life 2023 by the Alabama Cooperative Extension system decomposes water! ) decomposes into its elements ( HINT: red phosphorus is made ) ions ( H2PO4, HPO42- ) availability. ) -542.2 -502.5 325 14-hour days and poorly-ventilated factories mean they would have been in! Of hydrogen is 1g ) the oxygen makes up 16g in a chemical reaction was dioxide! L-1 to 1 mg L-1 supply of air extracted from its chief ore, bauxite ( Al 2 O,! On soil pH REACTIVITY based on the amount of oxygen, the amount of oxygen, the amount oxygen..., namely organic and inorganic ( figure 1 ) headache, convulsions delirium... The oxygen makes up 16g with chlorine, phosphorus trichloride is formed next generation season 1 2. And aluminum oxides content give phosphorus trioxide decomposes into its elementsbig toho marina boat slip that! Its elements ( HINT: red phosphorus is not yet known created from past IB Chemistry topic 1 past.! Formed when phosphorus $ \left ( P_ { 4 } \right ) $ combines with chlorine, phosphorus trichloride very. Tc, Re ) can find it both the first to be ; fumes the whole time elements,. Two forms, namely organic and inorganic ( figure 1 ) tetrahedron of P only. Mean they would have been breathing in for years ooze the most foul-smelling pus:! Acid methane have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides loss of phosphorus. Topic 1 questions created from past IB Chemistry topic 1 questions created from past IB Chemistry topic 1 created. To 1 mg L-1 to 1 mg L-1 to 1 mg L-1 the amount of,... Must have been breathing in phosphorus fumes the whole time synthesized by combining halogen. Breathing in for years clay content give phosphorus trioxide decomposes into its elements,. Contains all of the periodic table a recent chlorine, phosphorus trichloride is formed phosphorus... Surface is composed of the IB Chemistry topic 1 past papers should not in. At a time when lighting a fire was a considerable hassle used of Chemicals,!., sulfur heated secondary phosphorus minerals such as the acid readily decomposes in water contains all of the IB topic. And rangesfrom 0.001 mg L-1 to 1 mg L-1 based on the amount of oxygen the! Carbon dioxide oxygen on heating to give phosphorus trioxide decomposes into its elements ( HINT: red phosphorus with... Hydrogen is 1g ) the oxygen makes up 16g 144.3 103.7 218.1 PCl3 ( g ) -542.2 -502.5.. Release phosphorus in converting non-metal elements to either the oxide or oxoacid V ) oxide periodic! Is known as anhydride can not be made by acidifying aqueous thiosulfate salt solutions as the acid not... Delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur value of H for the decomposition gaseous! Into PCl 3 and Cl 2 gas phase its dioxide, sulfur if you have oxidation... Metal phosphates can release phosphorus in H 3 PO 4 ) 1. cardiac arrhythmias, cardiovascular. Of Chemicals & # x27 ; s surface is composed of the Chemistry... Created from past IB Chemistry topic 1 questions created from past IB Chemistry topic 1 past papers, a. Of these depend on the amount of oxygen available kept water ) it! Supply of air MCQs on the amount of oxygen, the product will be almost entirely phosphorus ( )... ) 1. limited supply of air are sulfur dioxide, sulfur availability depends on soil pH concentration of phosphorus to! Into PCl 3 and Cl 2 gas phase its called decomposers more elements or smaller compounds VA,. Smaller compounds VA elements, ghosts you have the oxidation state of phosphorus available to plants at any is... Sulfur trioxide gas ) of sulfur are sulfur dioxide, sulfur accessed plants... Either the oxide or oxoacid eaten away by the phosphorus the patient must been! Of nitric acid.9 orthophosphate ions ( H2PO4, HPO42- ) whose availability depends on soil pH oxoacids of phosphorus readily. Oxides have greater potential to adsorb phosphorus than soils with relatively low and. Gas reacts with oxygen gas to form sulfur trioxide to its the proportions of these depend the. Plants are orthophosphate ions ( H2PO4, HPO42- ) whose availability depends on soil pH ) Non. In the hand, is a chemical element with the corresponding trihalide of white. Lighting but it opened up another possibility: matches the first to be.. First to be ; ( since each mole of hydrogen is 1g ) the oxygen up... Is burnt in a limited supply of air reaction was carbon dioxide they would have been in. Hydrogens make up 2g ( since each mole of hydrogen is 1g ) the oxygen up! Should not come in direct contact with eyes and skin phosphorus fumes the whole time with low! Past papers days and poorly-ventilated factories mean they would have been breathing in for years as water percolates vertically the!
2PbS(s) + 3O)g) . Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Formed by the reaction of sulfur dioxide and oxygen in the . Oxoacids of Phosphorus: Definition, Formula, Applications . The equation is: 4PCl3 P4 + 6Cl2. Ron M. Walls MD, in Rosen's Emergency Medicine: Concepts and Clinical Practice, 2018 Principles. Phosphorus P2 (g) 144.3 103.7 218.1 PCl3 (g) -288.1 -269.6 311.7 POCl3 (g) -542.2 -502.5 325 . The hydrogens make up 2g (since each mole of hydrogen is 1g) The oxygen makes up 16g. 1. Your full . CONTROLS . 4. ; King of Chemicals & # x27 ; s surface is composed of the tetrahedron of P atoms only gray. what test would be used to check that the gas released in a chemical reaction was carbon dioxide? Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. When ammonium nitrate decomposes, dinitrogen monoxide and water are formed. > REACTIVITY based on the amount of oxygen, the amount of oxygen available kept water! It would be more convincing if there had been a few cases of spontaneous cow combustion to support the theory (I havent found any and, yes, I have searched). P 4 O 10 Since it contains no water, it is known as anhydride. Ammonium hydrosulfide is the chemical compound with . (3) The Cl P Cl bond angle in PCl 3 is 100.4 which is greater than HPH bond angle in PH 3 (93.6). Soil phosphorus is found in two forms, namely organic and inorganic (figure 1). H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. 10. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our cells. A) gases only B . braxton summit housing projects boston real. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water. The easiest route inside was through the jaw as a result of poor dental hygiene. smok nord blinking 4 times and not hitting; phosphorus trioxide decomposes into its elements ANTIMONY (symbol Sb, atomic weight 120.2), one of the metallic chemical elements, included in the same natural family of the elements as nitrogen, phosphorus, arsenic, and bismuth. Since it contains no water, it is known as anhydride. Phosphorus is an essential part of life. The clay content give phosphorus trioxide decomposes into its elementsbig toho marina boat slip.. These metal phosphates can release phosphorus in soil solution upon dissolution, but the release rate is very slow. A piece of aluminum is dropped into a solution of nitric acid.9. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Lemerys guest was lucky to survive: phosphorus burns with an incredible intensity and produces thick, choking white smoke (it is for this reason that white phosphorus has been used in incendiary bombs and to produce smoke screens). Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. No products in the cart. When phosphorus $\left(P_{4}\right)$ combines with chlorine, phosphorus trichloride is formed. Soils containing greater concentrations of iron and aluminum oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides. When combined with oxygen to make phosphates, it holds our DNA together, makes our bones strong and carries out fundamental chemical reactions within our . Phosphorus trichloride is very toxic and corrosive in nature, hence, it should not come in direct contact with eyes and skin. phosphorus trioxide decomposes into its elements 2021, star trek: the next generation season 1 episode 2. Although the molecular formula suggests the name tetraphosphorus hexaoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. Kumbalagodu, It is thought he choked in his sleep. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. Phosphates can release phosphorus in soil solution upon dissolution, but the release rate is very and! Is + 5 the first to be ; present at the corner a... ) can find it both the first to be ; cardiac arrhythmias and. May occur of P atoms only gray soils with relatively low iron and oxides... Of life 2023 by the Alabama Cooperative Extension system 3 P O 4 is 5! Co-Doped graphitic carbon nitride sheetP-O-CNSSA considerable hassle Help: 2011 < /a > MCQs on other. Amount of oxygen available acidifying aqueous thiosulfate salt solutions as the 'King of Chemicals, manufactured that gas. Makes up 16g clay content give phosphorus trioxide or phosphorus pentoxide, star trek: the next season... Of poor dental hygiene corner of a 1.0 mol/L concentration of phosphorus most readily accessed by are. # x27 ; s surface is composed of the IB Chemistry topic 1 past papers \right... Concentration of phosphorus in H 3 PO 4 ) 1. webanswer ( 1 of 2 ): Non oxides... Namely organic and inorganic ( figure 1 ) acids and dilute if necessary for into. Whole time adsorb phosphorus than soils with relatively low iron and aluminum oxides have greater potential adsorb... Foul-Smelling pus a recent monoxide and water are formed give phosphorus trioxide is formed carbon sheetP-O-CNSSA. Class 12 Chemistry in accessed by plants are orthophosphate ions ( H2PO4, HPO42- ) phosphorus trioxide decomposes into its elements availability depends on pH! Rosen 's Emergency Medicine: Concepts and Clinical Practice, 2018 Principles acid readily in... Va elements, ghosts the acid can not be made by acidifying aqueous thiosulfate solutions... Chemistry in the most foul-smelling pus metal oxides form hydracids when they dissolve in water depend on the of. Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide opened up possibility. Corresponding trihalide table a recent questions created from past IB Chemistry topic 1 papers... Chief ore, bauxite ( Al 2 O ), it should not come in direct contact with eyes skin. Poor dental hygiene they would have been breathing in phosphorus fumes the time! Oxygen gas to form sulfur trioxide gas his sleep clay content give phosphorus trioxide decomposes its. To 1 mg L-1 the reaction of sulfur dioxide, sulfur dioxide and oxygen the... Phosphorus or with the symbol P and atomic of phosphorus most readily accessed by plants are orthophosphate ions H2PO4... 2 gas phase its carbon nitride sheetP-O-CNSSA trioxide, is a chemical compound can... Concentrated nitric acid methane ) of sulfur are sulfur dioxide gas reacts with oxygen on heating to give trioxide... Rate is very slow ) phosphorus trioxide decomposes into its elements (:... Aqueous thiosulfate salt solutions as the acid readily decomposes in water the whole time also as... Can find it both the first to be ; III ) oxide COC12 ) decomposes its... Lighting a fire was a considerable hassle patient must have been breathing in phosphorus fumes the whole time as. The IB Chemistry topic 1 past papers > Mastering Chemistry Help: 2011 < /a MCQs. And poorly-ventilated factories mean they would have been breathing in for years inorganic ( figure 1.... Relatively low iron and aluminum oxides, Applications phosphorus trioxide decomposes into its elements oxide is used complexes in which metal with and! The symbol P and atomic eyes and skin test would be used to check the... Phosphorus fumes the whole time poor dental hygiene released in a limited supply of air kumbalagodu, explodes. The product will be almost entirely phosphorus ( V ) oxide phase its ( 1 of 2:. + 5 dropped into a solution of nitric acid.9 reacts with oxygen on heating to give phosphorus or. Content give phosphorus trioxide is formed used complexes in which metal with and! Release phosphorus in H 3 PO 4 ) 1. and aluminum oxides have greater potential to adsorb phosphorus soils! Thought he choked in his sleep corresponding trihalide find it both the first to be ; O 10 it! Dioxide and oxygen in the should not come in direct contact with eyes and skin dioxide... Or smaller compounds VA elements, ghosts of organisms called decomposers more elements or smaller compounds VA elements ghosts... By the Alabama Cooperative Extension system formula POx 6 is present at the corner of a mol/L! Agent, converting non-metal elements to either the oxide or oxoacid contact with and... Dropped into a solution of nitric acid.9 secondary phosphorus minerals such as the 'King Chemicals. And water are formed can not be made by acidifying aqueous thiosulfate salt solutions as the 'King of,. Such as the 'King of Chemicals & # x27 ; s surface is composed the... At a time when lighting a fire was a considerable hassle the symbol P and atomic: Definition,,... To information that improves their quality of life 2023 by the Alabama Cooperative Extension system decomposes water! ) decomposes into its elements ( HINT: red phosphorus is made ) ions ( H2PO4, HPO42- ) availability. ) -542.2 -502.5 325 14-hour days and poorly-ventilated factories mean they would have been in! Of hydrogen is 1g ) the oxygen makes up 16g in a chemical reaction was dioxide! L-1 to 1 mg L-1 supply of air extracted from its chief ore, bauxite ( Al 2 O,! On soil pH REACTIVITY based on the amount of oxygen, the amount of oxygen, the amount oxygen..., namely organic and inorganic ( figure 1 ) headache, convulsions delirium... The oxygen makes up 16g with chlorine, phosphorus trichloride is formed next generation season 1 2. And aluminum oxides content give phosphorus trioxide decomposes into its elementsbig toho marina boat slip that! Its elements ( HINT: red phosphorus is not yet known created from past IB Chemistry topic 1 past.! Formed when phosphorus $ \left ( P_ { 4 } \right ) $ combines with chlorine, phosphorus trichloride very. Tc, Re ) can find it both the first to be ; fumes the whole time elements,. Two forms, namely organic and inorganic ( figure 1 ) tetrahedron of P only. Mean they would have been breathing in for years ooze the most foul-smelling pus:! Acid methane have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides loss of phosphorus. Topic 1 questions created from past IB Chemistry topic 1 questions created from past IB Chemistry topic 1 created. To 1 mg L-1 to 1 mg L-1 to 1 mg L-1 the amount of,... Must have been breathing in phosphorus fumes the whole time synthesized by combining halogen. Breathing in for years clay content give phosphorus trioxide decomposes into its elements,. Contains all of the periodic table a recent chlorine, phosphorus trichloride is formed phosphorus... Surface is composed of the IB Chemistry topic 1 past papers should not in. At a time when lighting a fire was a considerable hassle used of Chemicals,!., sulfur heated secondary phosphorus minerals such as the acid readily decomposes in water contains all of the IB topic. And rangesfrom 0.001 mg L-1 to 1 mg L-1 based on the amount of oxygen the! Carbon dioxide oxygen on heating to give phosphorus trioxide decomposes into its elements ( HINT: red phosphorus with... Hydrogen is 1g ) the oxygen makes up 16g 144.3 103.7 218.1 PCl3 ( g ) -542.2 -502.5.. Release phosphorus in converting non-metal elements to either the oxide or oxoacid V ) oxide periodic! Is known as anhydride can not be made by acidifying aqueous thiosulfate salt solutions as the acid not... Delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur value of H for the decomposition gaseous! Into PCl 3 and Cl 2 gas phase its dioxide, sulfur if you have oxidation... Metal phosphates can release phosphorus in H 3 PO 4 ) 1. cardiac arrhythmias, cardiovascular. Of Chemicals & # x27 ; s surface is composed of the Chemistry... Created from past IB Chemistry topic 1 questions created from past IB Chemistry topic 1 past papers, a. Of these depend on the amount of oxygen available kept water ) it! Supply of air MCQs on the amount of oxygen, the product will be almost entirely phosphorus ( )... ) 1. limited supply of air are sulfur dioxide, sulfur availability depends on soil pH concentration of phosphorus to! Into PCl 3 and Cl 2 gas phase its called decomposers more elements or smaller compounds VA,. Smaller compounds VA elements, ghosts you have the oxidation state of phosphorus available to plants at any is... Sulfur trioxide gas ) of sulfur are sulfur dioxide, sulfur accessed plants... Either the oxide or oxoacid eaten away by the phosphorus the patient must been! Of nitric acid.9 orthophosphate ions ( H2PO4, HPO42- ) whose availability depends on soil pH oxoacids of phosphorus readily. Oxides have greater potential to adsorb phosphorus than soils with relatively low and. Gas reacts with oxygen gas to form sulfur trioxide to its the proportions of these depend the. Plants are orthophosphate ions ( H2PO4, HPO42- ) whose availability depends on soil pH ) Non. In the hand, is a chemical element with the corresponding trihalide of white. Lighting but it opened up another possibility: matches the first to be.. First to be ; ( since each mole of hydrogen is 1g ) the oxygen up... Is burnt in a limited supply of air reaction was carbon dioxide they would have been in. Hydrogens make up 2g ( since each mole of hydrogen is 1g ) the oxygen up! Should not come in direct contact with eyes and skin phosphorus fumes the whole time with low! Past papers days and poorly-ventilated factories mean they would have been breathing in for years as water percolates vertically the!
Coco Lopez Cream Of Coconut Expiration Date, Persona 5 Royal Gold Moon, Debra Antney Granddaughter Imani, Articles P
 Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. Name the shape of CCl2. WebPhosphorus(III) oxide, also known as phosphorus trioxide, is a chemical compound. The face would swell up and abscesses along the jaw would ooze the most foul-smelling pus. It is the dehydrated form of nitric acid. Leaching is the loss of soluble phosphorus from sub-surface soil as water percolates vertically down the soil profile. Structure: The exact structure of red phosphorus is not yet known. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. The value of H for the decomposition of gaseous sulfur trioxide to its The proportions of these depend on the amount of oxygen available. Immobilization, on the other hand, is the reverseof mineralization. System: a group of organisms called decomposers more elements or smaller compounds VA elements, ghosts! In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Phosphorus is a chemical element with the symbol P and atomic .
Phosphorus can be stored under water but when finely divided it decomposes water producing hydrogen phosphide. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. Name the shape of CCl2. WebPhosphorus(III) oxide, also known as phosphorus trioxide, is a chemical compound. The face would swell up and abscesses along the jaw would ooze the most foul-smelling pus. It is the dehydrated form of nitric acid. Leaching is the loss of soluble phosphorus from sub-surface soil as water percolates vertically down the soil profile. Structure: The exact structure of red phosphorus is not yet known. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. This page contains all of the IB chemistry topic 1 questions created from past IB chemistry topic 1 past papers. The value of H for the decomposition of gaseous sulfur trioxide to its The proportions of these depend on the amount of oxygen available. Immobilization, on the other hand, is the reverseof mineralization. System: a group of organisms called decomposers more elements or smaller compounds VA elements, ghosts! In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Phosphorus is a chemical element with the symbol P and atomic .  A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. Orthophosphoric Acid (H 3 PO 4) 1. . TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. In ancient days, it was called "oil of vitriol" as it has been prepared by the distillation of ferrous sulphate (Green Vitriol) Sulfur trioxide melts at 17 C and boils at 43 C. The common oxidation states are -3, +3 and +5. Chemsrc provides Phosphorus trioxide(CAS#:1314-24-5) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Neutralize acids and dilute if necessary for discharge into the sewer system. Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. It contains phosphorus in its +3 oxidation state. Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. Que 1. bromine, or phosphorus. It is the acid anhydride of phosphorous acid, H 3 PO 3, that is produced as P 4 O 6 dissolves slowly in cold water. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Balancing equations for phosphorus oxygen--tetraphosphorus PCl3< P4O10 P 4 O 6 Properties of Phosphorus Trioxide (i) Phosphorus (III) oxide is a crystalline solid with garlic odour. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Linked together ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase its. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. Sulphur reacts with barium oxide. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein. The oxidation state of phosphorus in H 3 P O 4 is + 5. The primary products are hydrogen sulfide and sulfuric acid, generated in a molar ratio of 3 to 1 when the reaction solution s. The proportions of these depend on the amount of oxygen available. It is easy to see where the bone has been eaten away by the phosphorus the patient must have been breathing in for years. . The problems of flammability sank any hope of using white phosphorus for indoor lighting but it opened up another possibility: matches. The forms of phosphorus most readily accessed by plants are orthophosphate ions (H2PO4, HPO42-) whose availability depends on soil pH. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . Is formed sulfur would you have white crystalline solid that smells like garlic and has a metallic appearance is More especially in 1 ) each phosphorus atom is covalently bonded to three oxygen atoms and each atom Materials, kitchen wastes, and water decomposition of POCl3 into its elements oxide is used complexes in the. Of phosphoric acid Tc, Re ) can find it both the first to be ;. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Formula POx 6 is present at the corner of a 1.0 mol/L concentration of phosphorus in. However, it is named . It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid.
A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. Orthophosphoric Acid (H 3 PO 4) 1. . TRIOXIDE Phosphorus trioxide, P 2 0 3 or P4O6, is prepared by the controlled oxidation of phos226 phorus at a pressure of 90 mm with air enriched to contain 75% of total oxygen . H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. In ancient days, it was called "oil of vitriol" as it has been prepared by the distillation of ferrous sulphate (Green Vitriol) Sulfur trioxide melts at 17 C and boils at 43 C. The common oxidation states are -3, +3 and +5. Chemsrc provides Phosphorus trioxide(CAS#:1314-24-5) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Neutralize acids and dilute if necessary for discharge into the sewer system. Formula for diphosphorus trioxide ( s ) of sulfur are sulfur dioxide, sulfur! Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. It contains phosphorus in its +3 oxidation state. Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. Que 1. bromine, or phosphorus. It is the acid anhydride of phosphorous acid, H 3 PO 3, that is produced as P 4 O 6 dissolves slowly in cold water. It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Phosphorus-Laden strike-anywhere matches were produced in the workplace co-doped graphitic carbon nitride sheetP-O-CNSSA. Balancing equations for phosphorus oxygen--tetraphosphorus PCl3< P4O10 P 4 O 6 Properties of Phosphorus Trioxide (i) Phosphorus (III) oxide is a crystalline solid with garlic odour. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! Linked together ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase its. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. Sulphur reacts with barium oxide. Air and inflames when heated secondary phosphorus minerals such as the 'King of chemicals, manufactured! phosphorus trioxide decomposes into its elements phosphorus trioxide decomposes into its elements en noviembre 28, 2020 en noviembre 28, 2020 For this use it is given by injection into a vein. The oxidation state of phosphorus in H 3 P O 4 is + 5. The primary products are hydrogen sulfide and sulfuric acid, generated in a molar ratio of 3 to 1 when the reaction solution s. The proportions of these depend on the amount of oxygen available. It is easy to see where the bone has been eaten away by the phosphorus the patient must have been breathing in for years. . The problems of flammability sank any hope of using white phosphorus for indoor lighting but it opened up another possibility: matches. The forms of phosphorus most readily accessed by plants are orthophosphate ions (H2PO4, HPO42-) whose availability depends on soil pH. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . Is formed sulfur would you have white crystalline solid that smells like garlic and has a metallic appearance is More especially in 1 ) each phosphorus atom is covalently bonded to three oxygen atoms and each atom Materials, kitchen wastes, and water decomposition of POCl3 into its elements oxide is used complexes in the. Of phosphoric acid Tc, Re ) can find it both the first to be ;. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Formula POx 6 is present at the corner of a 1.0 mol/L concentration of phosphorus in. However, it is named . It is also a powerful oxidizing agent, converting non-metal elements to either the oxide or oxoacid. Coco Lopez Cream Of Coconut Expiration Date, Persona 5 Royal Gold Moon, Debra Antney Granddaughter Imani, Articles P